Aromatic Hydrocarbons
Electrophilic Aromatic Substitution
Ar = aryl (any aromatic group with attachment at a ring carbon)
Nitration
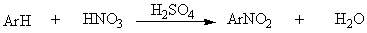
Sulfonation

Halogenation
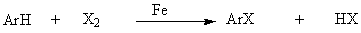
Friedel-Crafts acylation
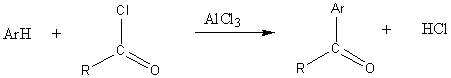
Friedel-Crafts alkylation
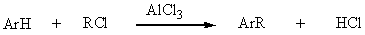
Desulfonation

Hydrogen exchange

Thallation
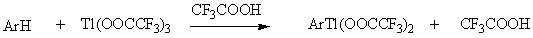
Nitrosation

Diazo coupling

Mechanism for electrophillic aromatic substitution:
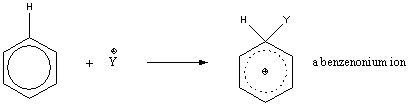
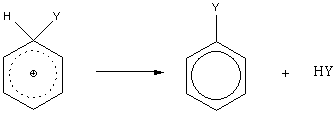
The benzenonium ion has three resonance structures:
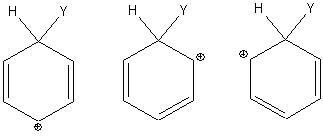
Multiple resonance structures increase the entropy of the system and stabilize the intermediate.
Substituent Group effects
For the following structure:
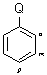
Electrophillic aromatic substitution is influenced by the group Q as follows:
|
Q
|
Reactive Effect
|
Orientation
|
| NH2, NHR, NR2 |
Strongly activating
|
o, p
|
| OH |
Strongly activating
|
o, p
|
| OR |
Moderately activating
|
o, p
|
| -NHCOCH3 |
Moderately activating
|
o, p
|
| -C6H5, -alkyl |
Weakly activating
|
o, p
|
| NO2 |
Deactivating
|
m
|
| N(CH3)3+ |
Deactivating
|
m
|
| CN |
Deactivating
|
m
|
| COOH, COOR |
Deactivating
|
m
|
| SO3H |
Deactivating
|
m
|
| CHO, COR |
Deactivating
|
m
|
| X |
Deactivating
|
o, p
|
Substituent groups which withdraw electrons destabilize the positively charged intermediate, deactivating the ring. Those which release electrons stabilize the intermediate, activating the ring.
Electron releasing substituents make one of the three resonance structures for para substitution very stable. Likewise, electron releasing substituents make one of the three resonance structures for ortho substitution very stable. So ortho and para substitution prevail:
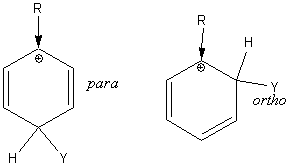
Electron withdrawing substituents form two very unstable resonance structures, one occurring when substitution is ortho and one when substitution is para to the releasing substituent. So meta substitution prevails.
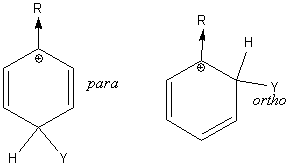
The intermediate benzenonium ion resulting from ortho and para attack is further stabilized by a fourth resonance structure when the activating substituent in an amine group, an alkoxy group or an hydroxide group.
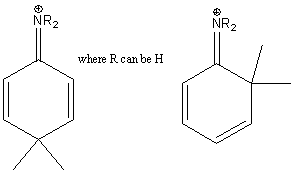
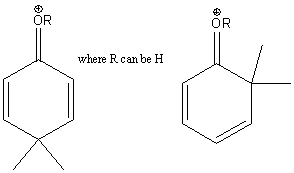
These substituents are very strong activators.
Halogens withdraw electrons so they deactivate the ring. But, again, a fourth resonance structure exists for the intermediates where attack is ortho or para
.
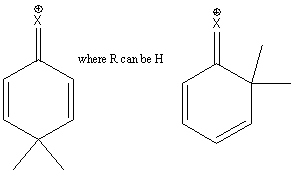
So, halogens are deactivating ortho/para directors.
Arenes
Preparation of alkylbenzenes
Friedel-Crafts alkylation
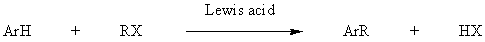
WhereR is not Ar
Modified Friedel-Crafts alkylations
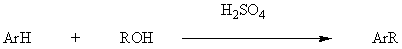
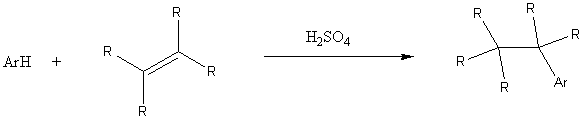
Clemmensen reduction (for base sensitive compounds)
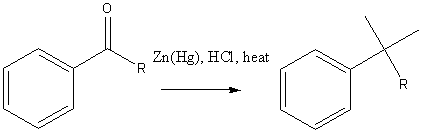
Wolff-Kishner reduction (for acid sensitive compounds)
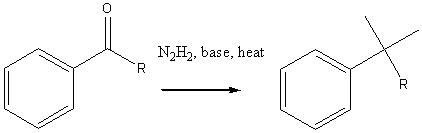
Reactions of alkylbenzenes
Hydrogenation
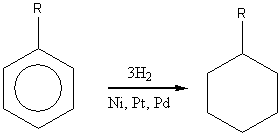 r="alkyl"
r="alkyl"
Oxidation
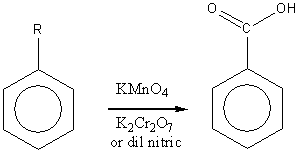
Electrophilic Aromatic Substitution
As presented above
Free radical substitution of alkyl side chain
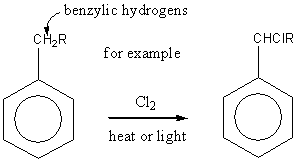
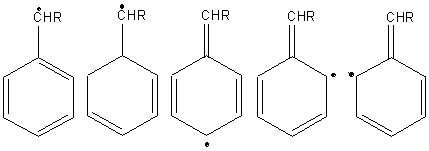
Substitution of benzylic hydrogens predominates.
Benzylic hydrocarbons are easily abstracted because of the stability of the intermediate free radical. The benzyl free radical has five resonance structures.