α, β - Unsaturated Carbonyl Compounds
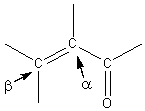
Carbon-carbon double bond is deactivated towards electrophilic addition by the electron withdrawing carbonyl group. However, the double bond is activated towards nucleophilic addition. In electrophilic addition the intermediate is a cation. In nucleophilic addition the intermediate is an anion. Addition to the carbon-carbon double bond takes place with orientation of the negative addition species adding to the β-carbon and the positive species adding to the α-carbon.
Electrophilic addition

Nucleophilic addition

The Michael Addition
The Michael addition involves the formation of a carbanion through the abstraction of the acidic hydrogen (by a base) from the following three compounds.
Ethyl malonate:

Ethyl acetoacetate

Ethyl cyanoacetate

The nucleophile thus formed can then combine with an α, β-unsaturated carbonyl compound. For example:

The Diels-Alder Reaction
A cycloaddition of a conjugated diene and an α, β-unsaturated carbonyl compound.

For example:

Quinones are an example of a highly conjugated α, β-unsaturated carbonyl compound: